Structures and properties of side-chain liquid crystalline polynorbornenes containing an amide group: hydrogen bonding interactions and spacer length effects†
Abstract
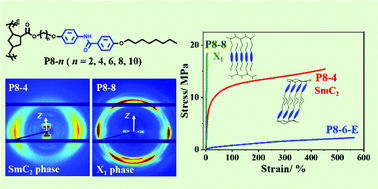
To investigate the structure–property relationship of side-chain liquid crystalline (LC) polymers with specific interactions, we synthesized a series of polynorbornene derivatives bearing benzanilide side-chains (denoted as P8-n, where n represents the number of methylene units in the spacer, and the number 8 indicates the side-chain tail of the octyl group). For comparison, a reference polynorbornene derivative (denoted as P8-6-E) was also synthesized by replacing the amide group in the rod-like mesogen with an ester group. It is found that with the polynorbornene main-chain the samples can exhibit rich LC behaviors, different from other benzanilide-containing polymers. P8-2 and P8-4 form a bilayer smectic C (SmC2) phase. On the other hand, with longer spacers, the molecules of P8-8 and P8-10 can pack into a highly ordered structure (denoted as X1). The X1 phase has an orthorhombic lattice with the side- and main-chains along the c- and b-axes, wherein the side-chains show interdigitated packing with some features of a crystal E (CrE) structure. For P8-6, some bilayer CrE domains may coexist with X1, resulting in a mixed phase of X1/E2. It is unveiled that the amide group at the center of benzanilide always tends to form hydrogen bonds, leading to the unique molecular packing of P8-ns which is dependent on the size matching between the spacer and tail. Moreover, weakening the hydrogen bonds in P8-ns (n ≥ 8) could induce a phase transition from X1 to X1/E2. Compared with the rather soft and ductile P8-6-E with a smectic B phase, P8-ns show much higher Young's moduli because of the presence of lateral hydrogen bonds. While those with the X1 phase are brittle, the P8-ns with a SmC2 structure exhibit better overall mechanical properties, rendering a breaking strain of ∼450%.


 Please wait while we load your content...
Please wait while we load your content...