Ring-expansion cationic cyclopolymerization for the construction of cyclic cyclopolymers†
Abstract
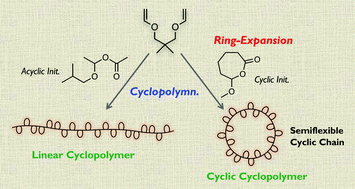
A “cyclic cyclopolymer” was successfully synthesized via ring-expansion cationic cyclopolymerization with a cyclic initiator by using a divinyl ether carrying a gem-dimethyl group on the spacer. The unprecedented polymerization was achieved by designing the monomer on the basis of the Thorpe–Ingold effect as well as optimizing the conditions for living cationic cyclopolymerization with the model “acyclic” initiator. The obtained cyclic cyclopolymer exhibited an unusual glass transition temperature (Tg) trend: its Tg decreased as the molecular weight was increased, and was lower than that of the linear cyclopolymer analogue. This unique feature presumably derived from conflicting influences: namely, on the one hand, a lowering of the Tg resulting from a larger free volume due to the cyclic topology; and, on the other hand, a raising of the Tg as a result of the semiflexible chain.


 Please wait while we load your content...
Please wait while we load your content...