Near-infrared light-responsive UCST-nanogels using an efficient nickel-bis(dithiolene) photothermal crosslinker†
Abstract
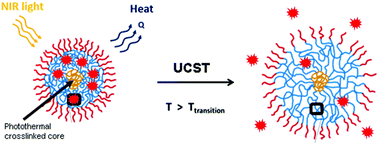
A new kind of near-infrared (NIR) light-responsive polymer nanogel is demonstrated. The micellar aggregates of an ABA-type triblock copolymer, whose core is a thermosensitive polymer displaying an upper critical solution temperature (UCST), are crosslinked using a photothermal nickel-bis(dithiolene) complex that absorbs NIR light and converts efficiently optical energy into heat. We show that when the nanogel aqueous solution is exposed to NIR light, even at a low power density of 0.16 W cm−2 and a low nickel-bis(dithiolene) complex concentration of 61.4 μg mL−1, the photothermally induced heating is sufficient to allow the nanogel particles to undergo a volume phase transition. The induced volume increase due to the positive thermosensitivity of the polymer leads to the release of loaded hydrophobic dye molecules. Using an energy balance model, the photothermal conversion efficiency of the nickel-bis(dithiolene) complex in the nanogel was evaluated through solution temperature and transmittance measurements under NIR laser irradiation at various light power densities as well as different nanoparticle concentrations and solvents. The photothermal conversion efficiency can reach about 64%, which positions the nickel-bis(dithiolene) complex among the most efficient photothermal agents in the NIR spectral region around 1000 nm.


 Please wait while we load your content...
Please wait while we load your content...