Allyl amino-thioxanthone derivatives as highly efficient visible light H-donors and co-polymerizable photoinitiators†
Abstract
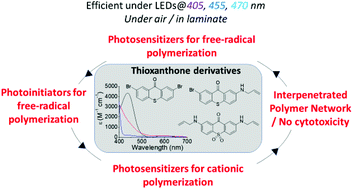
This study describes the role of two novel visible-light sensitive mono-allyl (TXA) and di-allyl sulfone (TXo2A) thioxanthone derivatives as photosensitizers and photoinitiators (PIs) for free-radical and cationic polymerizations (FRP and CP) and thiol–ene processes upon visible-light emitting diode exposure (LEDs@405 nm, 455 and 470 nm). The photoactivable groups incorporated into TXA and TXo2A have extended their absorption to the visible region up to 510 nm. TXo2A is the first example of a monomeric photoinitiator having two allyl side-chain groups. These new photoinitiating systems have first demonstrated unambiguously that they can be used as PIs and H donor molecules upon visible LED irradiation for the FRP of acrylates without additives. TXA and TXo2A also show highly efficient initiating properties when associated with judicious co-initiators i.e. an iodonium salt (Iod) as an electron acceptor, a tertiary amine (methyldiethanol amine, MDEA) as an electron donor, or a thiol derivative (trimethylolpropane tris (3-mercaptopropionate, TT) as a H-donor, under air and in laminate, for FRP, CP and thiol–ene reactions. These unprecedented initiating combinations promote high acrylate and epoxy conversions even under air, with a higher efficiency than the well-known CQ (and TX)-based photoinitiating systems used as references. As evidenced by nanosecond laser flash photolysis (LFP), electron paramagnetic resonance spin-trapping (EPR ST) and fluorescence/phosphorescence experiments, TXA (or TXo2A) can play a triple role, first as a proton/proton-coupled electron transfer initiator using MDEA or TT, as an electron donor when associated with Iod, and free-radical PIs following an intermolecular electron/proton transfer reaction. Interestingly, the photoinduced 3D interpenetrated polymer network (IPN) synthesized via the concomitant FRP and CP of an acrylate/epoxide monomer mixture upon LED@405 or 455 nm exposure has shown the capability to support viable cell proliferation without any cytotoxicity.


 Please wait while we load your content...
Please wait while we load your content...