Amide transformation as an efficient postpolymerization modification approach for the synthesis of functional polyacetylenes†
Abstract
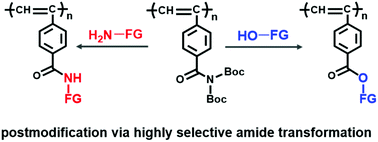
Postpolymerization modification based on selective amide transformation reactions is explored for the preparation of functional polymers, since amides are an important building motif ubiquitously present in biological and synthetic polymers. Inspired by recent advances in amide chemistry, a N,N-Boc2-benzamide group-containing polyacetylene (P1) was synthesized and used as a polymer substrate for polymer modification. Transamidation of P1 using amine nucleophiles, (R)- and (S)-1-phenylethanamine and anthracen-9-ylmethanamine, proceeded completely at 30 °C under metal-free conditions to produce polymers featuring predominantly one-handed helical structures and enhanced luminescence (P1a–P1c). The reaction with alcohols needed more forcing conditions (at 60 °C with DMAP as the catalyst) to eventually achieve full substitution. Incorporation of 2-(2-(2-methoxyethoxy)ethoxy)ethanol led to a dramatic increase in the surface energy of P1d. P1e obtained using 4-hydroxy-2,2,6,6-tetramethylpiperidine-1-oxyl exhibited reversible charge/discharge ability as a cathode material in a Li-ion battery.


 Please wait while we load your content...
Please wait while we load your content...