Evidence for ultrafast formation of tribenzoylgermyl radicals originating from tetraacylgermane photoinitiators†
Abstract
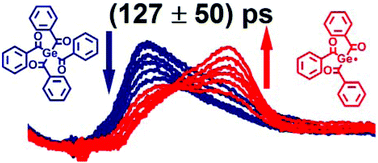
Mono-, bis-, and tetraacylgermane derivatives are promising photoinitiators for visible light-induced free radical polymerization. In the current study, the ultrafast dynamics of one unsubstituted as well as two ortho-methyl- and -ethyl-substituted tetraacylgermane derivatives were investigated in solution at ambient temperature via femtosecond-transient broadband (350–680 nm) absorption spectroscopy after 400 nm excitation. For the first time, we provide evidence for an initial formation of tribenzoylgermyl radical fragments originating from tetraacylgermane derivatives within 90–127 ps. By comparison to our previous quantitative pulsed laser polymerization and mass spectrometry study, the ultrafast radical formation can be related to the most efficient initiation ability compared to mono- and bis-derivatives.


 Please wait while we load your content...
Please wait while we load your content...