Dicarboxylic acid-epoxy vitrimers: influence of the off-stoichiometric acid content on cure reactions and thermo-mechanical properties†
Abstract
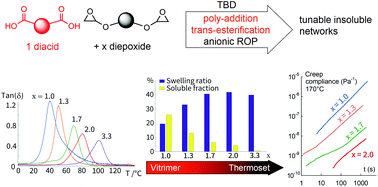
The present study explores a broad range of stoichiometry, with the [epoxy]/[acyl] ratio ranging from excess to unity for commercial diepoxide/sebacic acid vitrimer formulations, with 1,5,7-triazabicyclo[4.4.0]dec-5-ene (TBD) used as the catalyst. In particular, it investigates to what extent side reactions promoted by off-stoichiometry mixtures can help achieve desirable thermomechanical properties (i.e. glass transition Tα, Young's modulus, strain at break, and strength) for an optimised vitrimer that behaves like a stiff material at room temperature, retaining its capacity to flow at high temperature while remaining insoluble. The possible role of TBD as an anionic initiator is tested in the homopolymerisation of epoxy and compared to a known anionic initiator, 2-phenylimidazole (2-PI). Attenuated total reflection infrared (ATR-IR) spectroscopy reveals different reaction speeds, but an identical scenario for either 2-PI or TBD. The acid + epoxy addition occurs first, then epoxy homopolymerisation takes place after di-carboxylic acid consumption; an ester typically forms in less than 20 min at 125 °C with TBD, while the formation of ether takes several hours. For all [epoxy]/[acyl] ratios ranging from 1 : 1 to 1 : 0.3, it is found that the integrity of the network is retained when subjected to 1,2,4 trichlorobenzene (TCB) solvent treatment. From the 1 : 1 to 1 : 0.75 epoxy to acyl ratio, the material retains full ability to flow and relax stresses under thermal stimulation, showing a 10 fold increase in viscosity and unchanged activation energy of about 100 kJ mol−1. Beyond 1 : 0.6 stoichiometry, a gradual transition from vitrimer to non-exchangeable crosslinked materials is observed as these networks show only partial stress relaxation due to interpenetration in the polyether network.
- This article is part of the themed collection: Chemistry for Covalent Adaptable Polymer Networks


 Please wait while we load your content...
Please wait while we load your content...