From isoselectivity to syndioselectivity: Lewis base regulates stereochemistry in 2-vinylpyridine polymerization†
Abstract
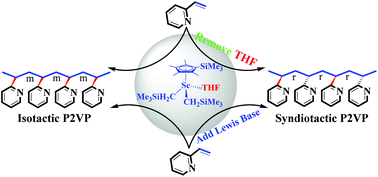
Poly(2-vinylpyridine) (P2VP) is a nitrogen-substituted analogue of polystyrene (PS). The first preparation of syndiotactic P2VP was realized using a (C5Me4SiMe3)Ln(CH2SiMe3)2(THF) (Ln = Sc, Y, Lu)/[Ph3C][B(C6F5)4] catalytic system for coordination polymerization of 2-vinylpyridine (2-VP). A strategy is reported for producing syndiotactic P2VP based on an isoselective catalytic system of 2-VP. The inhibition of THF coordination to the metal center of the catalyst shifts the stereocontrolled route to give syndiotactic P2VP, while catalysts with coordinated THF favor isotactic P2VP. Polymerization by the (C5Me4SiMe3)Sc(CH2SiMe3)2(THF)/[Ph3C][B(C6F5)4]/styrene catalytic system is the most stereoselective, yielding syndiotactic P2VP with syndioselectivity of Pr = 0.86. The Pr values of the polymers could be controlled in the range of 0.68 to 0.86 by adjusting the amount of Lewis base added. These syndiotactic polymers are crystalline materials, which displayed distinct melting point (Tm) values up to 138 °C.


 Please wait while we load your content...
Please wait while we load your content...