Self-crosslinking smart hydrogels through direct complexation between benzoxaborole derivatives and diols from hyaluronic acid†
Abstract
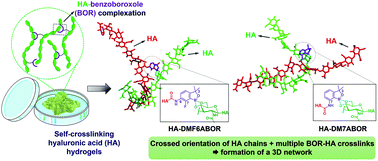
Boronate ester cross-linked hydrogels have emerged as promising injectable scaffolds for biomedical applications given their rapid self-healing ability. For a rational design of such networks, all variables influencing their dynamic rheological properties, especially the boronic acid and the diol-containing molecule selected as molecular crosslinkers have to be carefully considered. Herein, by tailoring the structure of benzoxaborole (BOR), self-crosslinking hydrogels based on hyaluronic acid (HA) modified with BOR derivatives are obtained for the first time through the direct BOR–HA diol complexation at physiological pH. Among the different HA–BOR conjugates investigated, those prepared from 6-amino-7-fluoro-3,3-dimethyl benzoxaborole (HA–DMF6ABOR) and 7-amino-3,3-dimethyl benzoxaborole (HA–DM7ABOR) show unprecedented self-crosslinking properties, leading to the formation of self-healing hydrogels with extremely slow dynamics. These networks also exhibit remarkable pH- and glucose-responsive behaviors. These properties are related to the peculiar structure of these two BOR moieties, having as the common feature, a gem-dimethyl group in the oxaborole ring and an ortho-substituent in the phenyl ring. Molecular dynamic simulations are used to provide insight in the role of these substituents in the outstanding capability of DMF6ABOR and DM7ABOR to crosslink HA. They show that BOR complexation induces changes in conformation of HA favoring formation of a highly entangled 3D network.


 Please wait while we load your content...
Please wait while we load your content...