Functionalisation and stabilisation of polymeric arsenical nanoparticles prepared by sequential reductive and radical cross-linking†
Abstract
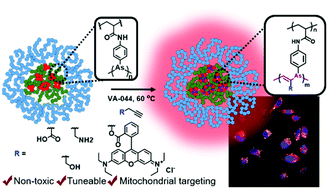
The chemical reactivity of arsenic is diverse and distinctive depending upon its interchangeable oxidation states. Alkyl and aryl arsines (As(I)) exist as oligomers, composed of labile and redox responsive As–As bonds which have been exploited to form reactive and responsive materials. Here, the lability and reactivity of As(I)-functional polymeric nanoparticles, derived from thermoresponsive polymers P(PEGA20-b-[NIPAm80-n-co-AsAmn]) (P1, n = 4; P2, n = 11; P3, n = 15; P4, n = 18), is elaborated by in situ reaction with functional acetylenes, resulting in the formation of vinylene–arsine cross-linked polymeric arsenical nanoparticles (NPV–As). Spherical particles with sizes <35 nm have been prepared, which are advantageous for potential drug-delivery (e.g. tumour accumulation) applications. Functional acetylenes enable the introduction of reactive amine, acid and alcohol functional groups into the particles, while the use of propargyl-O-rhodamine ester results in the formation of fluorescent nanoparticles. The vinylene–arsine cross-linking confers increased stability of the polymeric arsenical nanoparticles in model biological redox conditions (GSH, H2O2, 5 mM) compared to those reported previously, with nanoparticle structures retained over 7 days. The parent polymeric arsenicals and the resulting nanoparticles were all shown to exhibit limited cytotoxicity in vitro and cell uptake was confirmed by incubating fluorescent-labelled nanoparticles with PC3 cells. Furthermore, fluorescent confocal microscopy using the PC3 cell-line, confirmed that the nanoparticles were internalised by the cells with evidence of mitochondrial co-localisation, which supports a mitochondria-targeting of arsenic hypothesized based on work involving organoarsenical chemotherapeutics. Thus, this work demonstrates a novel strategy for the preparation of polymeric arsenical nanoparticles, with broad functional group tolerance, and expands our emerging understanding of the in vitro behaviour of this family of nanomaterials.


 Please wait while we load your content...
Please wait while we load your content...