Mono- and dimeric zinc(ii) complexes for PLA production and degradation into methyl lactate – a chemical recycling method†
Abstract
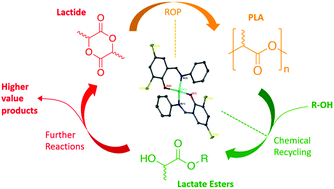
A series of well-defined mono- and dimeric Zn(II)-complexes were prepared and fully characterised by X-ray crystallography and NMR spectroscopy. Their application to the ROP of rac-LA to produce biocompatible atactic PLA was demonstrated in both solution and under industrially preferred melt conditions at 180 °C with a [rac-LA] : [Init] : [BnOH] of 3000 : 1 : 10. Exceptional activity was observed in all instances, albeit with generally poor molecular weight control (Mn) and broad dispersities (Đ), with dimers outperforming their monomeric counterparts. The propensity of all Zn(II)-complexes to facilitate PLA degradation into methyl lactate (Me-LA) under mild conditions is also demonstrated. Zn(2–3)2 were identified as the outstanding candidates, achieving full conversion to Me-LA within 8 h at 80 °C in THF. Further degradation kinetic analysis revealed Zn(2–3)2 to have kapp values of 0.63 ± 0.051 and 0.44 ± 0.029 h−1 for the rate of consumption of PLA respectively.


 Please wait while we load your content...
Please wait while we load your content...