Tacticity dependence of single chain polymer folding†
Abstract
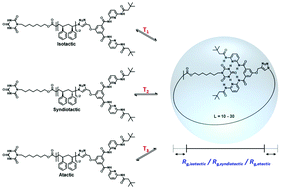
Precision polymerization techniques offer the exciting opportunity to manufacture single-chain nanoparticles (SCNPs) with intramolecular crosslinks placed in specific positions along the polymer chain. Earlier studies showed that synthetic polymer chains can fold into defined SCNP conformations through a reversible two-state process, similar to that observed for small peptides and proteins – yet far behind in its structural sophistication. While the natural structures of proteins arise from polypeptides of perfectly defined stereochemistry, the role of main-chain stereochemistry on SCNP folding remains largely unexplored. To investigate the effect of tacticity on SCNP architectures, the development of specific simulation strategies is critical to provide reliable data. Herein, we investigate the structural transitions of SCNPs of different stereochemistries, i.e. atactic, syndiotactic and isotactic of various lengths (L = 10 to L = 30) using all-atom Monte-Carlo simulations. The results indicate that structural transitions occur in syndiotactic polymers at lower temperature compared to atactic and isotactic polymer chains. The effect of main chain stereochemistry on the transition temperature was found to be especially pronounced for shorter polymer chains of length L = 10 to L = 20.


 Please wait while we load your content...
Please wait while we load your content...