Organocatalyzed ring-opening polymerization (ROP) of functional β-lactones: new insights into the ROP mechanism and poly(hydroxyalkanoate)s (PHAs) macromolecular structure†
Abstract
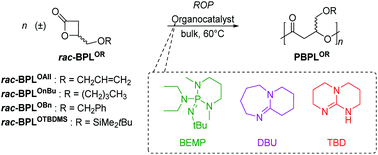
The organocatalyzed ring-opening polymerization (ROP) of various 4-alkoxymethylene-β-propiolactones (BPLORs; R = CH2CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2 (All), CH2Ph (Bn), (CH2)3CH3 (nBu), SiMe2tBu (TBDMS)) towards the formation of the corresponding poly(hydroxyalkanoate)s (PHAs; poly(BPLOR)s (PBPLORs)) is investigated under mild operating conditions (neat, 60 °C), simply using basic organocatalysts of the guanidine (1,5,7-triazabicyclo[4.4.0]dec-5-ene, TBD), amidine (1,8-diazabicyclo[5.4.0]-undec-7-ene, DBU) or phosphazene (2-tert-butylimino-2-diethylamino-1,3-dimethylperhydro-1,3,2diazaphosphorine, BEMP) type. The polymerization proceeds basically at the same rate as the alike organocatalyzed ROP of related β-lactones (especially the ubiquitous β-butyrolactone (BL) and alkyl β-malolactonates (MLARs)), with BEMP being significantly more active than TBD and DBU. Insights into the polymerization mechanisms are gained through detailed 1D/2D NMR spectroscopy and MALDI-ToF mass spectrometry analyses of the resulting PBPLORs and in particular through the identification of the nature of the end-capping groups. Each of the three organobases promotes the polymerization in its own way, as dictated by either its basic, nucleophilic or dual behavior.
CH2 (All), CH2Ph (Bn), (CH2)3CH3 (nBu), SiMe2tBu (TBDMS)) towards the formation of the corresponding poly(hydroxyalkanoate)s (PHAs; poly(BPLOR)s (PBPLORs)) is investigated under mild operating conditions (neat, 60 °C), simply using basic organocatalysts of the guanidine (1,5,7-triazabicyclo[4.4.0]dec-5-ene, TBD), amidine (1,8-diazabicyclo[5.4.0]-undec-7-ene, DBU) or phosphazene (2-tert-butylimino-2-diethylamino-1,3-dimethylperhydro-1,3,2diazaphosphorine, BEMP) type. The polymerization proceeds basically at the same rate as the alike organocatalyzed ROP of related β-lactones (especially the ubiquitous β-butyrolactone (BL) and alkyl β-malolactonates (MLARs)), with BEMP being significantly more active than TBD and DBU. Insights into the polymerization mechanisms are gained through detailed 1D/2D NMR spectroscopy and MALDI-ToF mass spectrometry analyses of the resulting PBPLORs and in particular through the identification of the nature of the end-capping groups. Each of the three organobases promotes the polymerization in its own way, as dictated by either its basic, nucleophilic or dual behavior.


 Please wait while we load your content...
Please wait while we load your content...