Poly(p-phenylene)s tethered with oligo(ethylene oxide): synthesis by Yamamoto polymerization and properties as solid polymer electrolytes†
Abstract
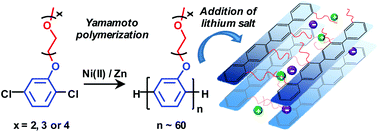
Salt-containing supramolecular assemblies of rigid-rod polymers tethered with flexible ion-solvating side chains represent a synthetic pathway towards thin ion-conducting solid electrolyte membranes with high dimensional stability. In the present work we have synthesized poly(p-phenylene)s (PpPs) carrying di-, tri- and tetra(ethylene oxide) side chains, respectively. p-Dichlorophenyl oligo(ethylene oxide) monomers were polymerized by Ni-mediated Yamamoto polymerization via in situ reduction of Ni(II). This gave PpPs with an average degree of polymerization reaching 60, where each phenylene ring carried one oligo(ethylene oxide) side chain. Results from calorimetry and X-ray scattering measurements clearly showed the formation of molecular composites, i.e., bicontinuous morphologies with mechanically reinforcing layers of the stiff PpP backbones separated by the flexible oligo(ethylene oxide) side chains. This morphology was retained after adding lithium bis(trifluoromethane)sulfonimide (LiTFSI) to form salt-in-polymer electrolytes, but with an increased distance between adjacent backbones. Furthermore, upon addition of salt the order-to-disorder transition (ODT) region increased from ∼50–170 °C to ∼75–200 °C at [EO]/[Li] = 20. Increasing salt concentrations also revealed a maximum in the ODT enthalpy at [EO]/[Li] = 40. At 80 and 160 °C, the ionic conductivity reached 1.1 × 10−4 and 1.0 × 10−3 S cm−1, respectively. Finally, we demonstrate that ionic conductivity of the polymer electrolytes can be significantly increased by additions of triglyme.


 Please wait while we load your content...
Please wait while we load your content...