Cyclo-oligomerization of hydroxyl-containing mono-functional benzoxazines: a mechanism for oligomer formation†
Abstract
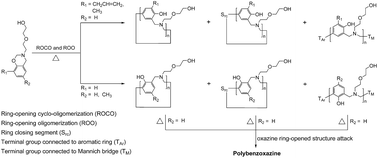
The oligomerization of mono-functional benzoxazines is demonstrated with intermolecular cyclization via a Mannich bridge structure as the primary reaction pathway. The structures of oligomers of mono-functional benzoxazines were previously thought to be benzoxazine dimers stabilized by inter- and intramolecular hydrogen bonding. This study is based on four hydroxyl-containing mono-functional benzoxazines synthesized from phenol, o-allylphenol, o-cresol, p-cresol, diglycolamine, and paraformaldehyde. A reaction mechanism is proposed that ortho- and para-substituted-phenol-based mono-functional benzoxazines undergo thermally activated ring-opening cyclo-oligomerization (ROCO) and ring-opening oligomerization (ROO), whereas their phenol-based counterpart polymerizes into polybenzoxazine following ROCO and ROO, which is evidenced by the data of 1H and 13C nuclear magnetic resonance spectra, Fourier transform infrared spectra, size exclusion chromatograms, and mass spectra of thermally treated samples.


 Please wait while we load your content...
Please wait while we load your content...