Polyesters with main and side chain phosphoesters as structural motives for biocompatible electrospun fibres†
Abstract
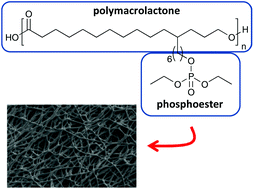
Phosphoester containing polymers are promising materials in biomedical applications due to their biocompatibility and biodegradability. Utilising thiol–ene chemistry, the synthesis of two novel structural polymer motives combining polyesters and phophoester groups was explored. The first polymer was obtained by coupling ene-functional poly(thioether-phosphoester) with thiol functional poly(pentadecalactone). While the coupling reaction was successful, yields remained low presumably due to inadequate endgroup stoichiometry. The second polymer comprised phosphoester side groups conjugated to unsaturated poly(globalide). Double bond conversions up to 84% were achieved depending of the type of phosphoester thiol and relative reactant ratios. The resulting polymers transitioned from solid semicrystaline to liquid amorphous with increasing degree of phosphoester conjugation. Electrospun fibres from polymers with 14% phosphoester conjugation allowed attachment and survival of human dermal fibroblasts, indicating their biocompatibility. These polymers represent a new class of easily accessible biocompatible polyester–phosphoester hybrid materials as potential building blocks for tunable biomaterials.


 Please wait while we load your content...
Please wait while we load your content...