Diselenide-crosslinked zwitterionic nanogels with dual redox-labile properties for controlled drug release†
Abstract
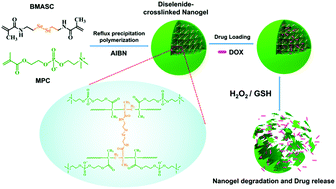
Drug delivery systems developed from zwitterionic polymers have intriguing antifouling properties. Combining these with stimuli-responsiveness, we fabricated a new type of zwitterionic nanogel with dual redox-labile properties by copolymerization of 2-methacryloyloxyethyl phosphorylcholine (MPC) and a diselenide bond-containing crosslinker through reflux precipitation polymerization. Poly(2-methacryloyloxyethyl phosphorylcholine) (PMPC)-based nanogels show high protein adsorption resistibility and colloidal stability at high salt concentrations. By incorporation of diselenide bonds into polymer networks, the nanogels are endowed with unique dual redox-labile properties that lead to their efficient degradation into short polymers either in a reducing environment (GSH) or in an oxidative environment (H2O2). Nanogels loaded with anticancer drugs (doxorubicin, DOX) display a finely controlled release behavior and low leakage of DOX under physiological conditions (15.0% in 24 h), but rapid and sufficient release under reducing conditions (88.3% in 24 h) or under oxidative conditions (82.2% in 24 h). Cell viability assays reveal that the blank nanogels have no cytotoxicity within a wide concentration range, while DOX-loaded nanogels present a significant inhibitive effect against tumor cells.


 Please wait while we load your content...
Please wait while we load your content...