Engineering digital polymer based on thiol–maleimide Michael coupling toward effective writing and reading†
Abstract
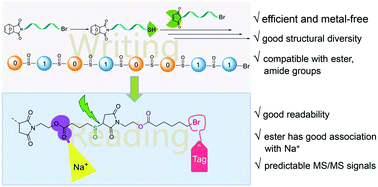
Digital polymers as promising molecule-level storage media have aroused more and more interest. The application of digital polymers strongly relies on efficient message writing and reading. Herein, we report the rational design of a digital polymer based on thiol–maleimide Michael coupling. Bromine was used as the precursor of the thiol group via thiourea/bromine nucleophilic substitution. This chemistry enabled efficient writing with excellent group tolerance and structural diversity. Importantly, the terminal bromine acting as an isotope tag enabled efficient reading by tandem mass spectrometry (MS/MS) sequencing. Moreover, to produce a more predictable tandem MS-induced fragmentation pattern, a computer simulation of the bond dissociation energy (BDE) was used. It was found that when the sulfur was oxidized to sulfoxide, the carbon–sulfur bond adjacent to the succinimide group had a much lower BDE, ∼32.2 kcal mol−1, allowing selective chain cleavage during MS/MS sequencing. The MS/MS sequencing of the sulfoxide–succinimide-linked digital polymer enabled efficient reading by creating an easily readable and predictable MS fragmentation pattern. The engineered digital polymers were endowed with both efficient writing and reading, which advanced the research and application of digital polymers.


 Please wait while we load your content...
Please wait while we load your content...