Comparison of poly(ethylene glycol)-based networks obtained by cationic ring opening polymerization of neutral and 1,2,3-triazolium diepoxy monomers†
Abstract
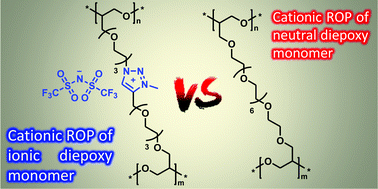
Poly(1,2,3-triazolium)s are a versatile class of poly(ionic liquid)s that take advantage of the functional tolerance, orthogonal robustness and efficiency of the copper(I)-catalyzed azide–alkyne cycloaddition (CuAAC). We use this reaction to design an all-in-one monomer gathering polymerization, crosslinking and ion-conducting functionalities in a single small molecule. A diepoxy 1,2,3-triazolium (DET) ionic liquid monomer is synthetized by CuAAC ligation between alkyne- and azide-functionalized epoxies, followed by N-alkylation of the central 1,2,3-triazole group by N-methyl bis(trifluoromethylsulfonyl)imide. Advantageously, this monomer is a low viscosity liquid which can therefore be implemented by casting. As a mode of curing, we chose cationic homopolymerization in bulk to obtain readily a network without dilution by a comonomer or release of byproducts. Two cross-linked epoxy networks were thereby obtained (i) from DET and (ii) from a commercial poly(ethylene glycol)diglycidyl ether (PEGDGE, Mn = 500 g mol−1), taken as a neutral reference monomer, using benzylamine trifluoroborate as cationic initiator. The polymerization kinetics and the structure/property correlations of the resulting ionic and neutral epoxy networks are discussed based on differential scanning calorimetry, thermogravimetric analysis and swelling/extractible measurements, as well as thermomechanical properties obtained by torsional rheometry and ionic conductivity measured by broadband dielectric spectroscopy. Although the ionic epoxy network exhibits a lower cross-link density (i.e. higher swelling ratio and lower storage modulus in the rubber state) than the neutral network due to the more pronounced occurrence of transfer and termination reactions, this work demonstrates that cationic ROP is a suitable route to produce a network solely from 1,2,3 triazolium functionalized diepoxy monomer. Besides, comparable levels of ionic conductivity were obtained for the neutral and 1,2,3-triazolium-based epoxy networks (i.e. σDC = 0.5 × 10−6 and 1 × 10−6 S cm−1 at 30 °C and under anhydrous conditions, respectively).


 Please wait while we load your content...
Please wait while we load your content...