Multifunctional tryptophan-based fluorescent polymeric probes for sensing, bioimaging and removal of Cu2+ and Hg2+ ions†
Abstract
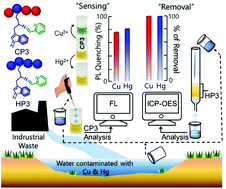
To develop a low-cost sensory assay capable of selective recognition with quantitative removal of toxic metal ions from an aqueous environment, we synthesized fluorescent polymeric probes fusing tryptophan and pyridine moieties in the side-chain of the polymer through an imine bond, with the aim of selectively detecting and separating both Cu2+ and Hg2+ ions from aqueous media. Amongst numerous metal ions, the water-soluble macromolecular probe selectively senses Cu2+ and Hg2+via 1 : 1 complex formation through two ‘N’ atoms of the pyridine moiety and imine group present in the side chain pendants of the probe, as supported by fluorescence and 1H NMR titration experiments. With Cu2+, a visible colour change from yellow to green was perceived that can be strategically used to differentiate between the two metal ions. This unique hydrophilic polymer is capable of sensing ppb levels of both Cu2+ and Hg2+ ions in water, below the contamination level recommended by the United States Environmental Protection Agency (EPA). Furthermore, intracellular imaging of Cu2+ and Hg2+ was also accomplished in neuroblastoma cells (U-87) with the biocompatible polymeric probe. Most importantly, around 97% removal of both metal ions from contaminated water was achieved using the hydrophobic homopolymer, as verified by inductively coupled plasma optical emission spectrometry (ICP-OES) analysis.


 Please wait while we load your content...
Please wait while we load your content...