Organocatalytic C–H fluoroalkylation of commodity polymers†
Abstract
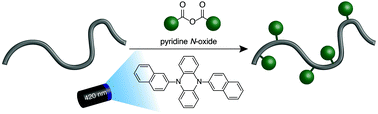
Post-polymerization modification of commodity plastics diversifies their material properties and expands their potential applications. The thermal and chemical stability of polymers that contain aromatic rings, which represent a high-volume class of materials, makes it challenging to add functionality while retaining the molar mass (Mn) and molecular weight distribution (MWD) of the original polymer. Herein, we present a metal-free C–H fluoroalkylation strategy that takes advantage of the innate reactivity of aromatic polymers. A systematic evaluation of organic photoredox catalysts for polymer C–H fluoroalkylation led to the identification of 5,10-di(2-naphthyl)-5,10-dihydrophenazine as a scaffold that balanced efficient reactivity with excellent selectivity, enabling the addition of trifluoromethyl groups onto aromatic polymers without altering the Mn or MWD of the material. The metal-free functionalization method was also amenable to a range of perfluoroalkyl groups and polymer substrates, including post-consumer and post-industrial plastic waste. The methods developed and structure–reactivity relationships identified in this study will prove valuable for the continued adoption of C–H functionalization methodologies that enhance the properties of commercially available polymers.
- This article is part of the themed collections: Polymer Chemistry Lectureship Winners and Plastics in a circular economy


 Please wait while we load your content...
Please wait while we load your content...