All-conjugated donor–acceptor block copolymers featuring a pentafulvenyl-polyisocyanide-acceptor†
Abstract
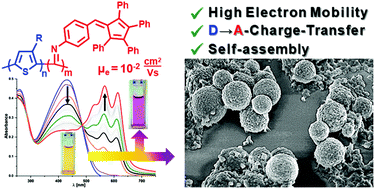
We report a fulvenyl-functionalized polyisocyanide (PIC2) with a high electron mobility of μe = 10−2 cm2 V−1 s−1. PIC2 has been incorporated into block-copolymers with either regioregular poly(3-dodecylthiophene) (P3DT → P(3DT-b-IC2)) or regioregular polythiazole (PTzTHX → P(TzTHX-b-IC2)). Block copolymer batches with different block-sizes have been isolated and their properties have been studied. Fluorescence quenching in the solid state and transient absorption spectroscopy indicate energy transfer from the donor- to the acceptor block upon photo-excitation. Fabrication of proof-of-principle organic photovoltaic cells with P(3DT-b-IC2) gave cells with an open circuit voltage (VOC) of ca. 0.89 V. The aggregation behavior of P(3DT-b-IC2) from solution was also studied, which revealed self-assembly into discreet microspheres of 1–8 μm diameter, with a size distribution of 1.72 (±0.37) μm under optimized aggregation conditions.


 Please wait while we load your content...
Please wait while we load your content...