Synthesis of polymeric topological isomers based on sequential Ugi-4CR and thiol–yne click reactions with sequence-controlled amino-functionalized polymers†
Abstract
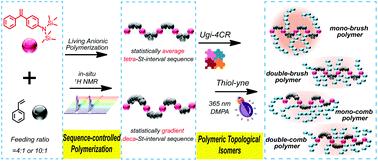
Herein, sequence-controlled polymerization and sequential Ugi-4CR/thiol–yne click reactions inspired a novel synthetic strategy for preparing a well-defined multigraft polymer in which the sequence-controlled amino-functionalized backbones were converted to mono- or di-modular alkynyl-functionalized main chains via an Ugi-4CR and clicked with well-defined thiol end-functionalized polystyrene branches through the “graft-onto” method. The Ugi-4CR showed high conversions (above 91%), while the efficiencies for the thiol–yne click reaction were above 97%. Meanwhile, an innovative concept of “polymeric topological isomers” that had similar molecular weights but different grafted structures was proposed for the first time. These four well-defined multigraft isomeric polymers (mono-brush polymer, double-brush polymer, mono-comb polymer and double-comb polymer) provide a high-level platform for polymer physics studies. Through preliminary measurements, the dilute solution properties were found to be significantly different and to change regularly with variations in the grafted structures.


 Please wait while we load your content...
Please wait while we load your content...