Regioselective, stereoselective, and living polymerization of divinyl pyridine monomers using rare earth catalysts†
Abstract
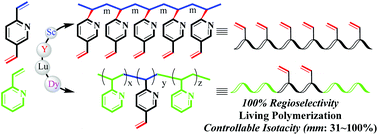
The first regioselective, stereoselective, and living polymerization of divinyl pyridine monomers, mediated by simple rare earth catalysts, is reported. The polymerization by Ln(CH2SiMe3)3(L)2 (Ln = Sc, Y, Lu, Dy; L = THF, Py) is perfectly regioselective for a 2,5-divinylpyridine (DVP) monomer, and the process only concerned the double bond at the 2-position of DVP and the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond at the 5-position selectively remained unreacted. In contrast, the polymerization of DVP by La(CH2SiMe3)3(THF)2 is not regioselective, producing a cross-linking network. The polymerization by Lu(CH2SiMe3)3(Py)2/B(C6F5)3 is most stereoselective, yielding perfect isotactic PDVP with mmmm >99%. The isoselectivity (mm) of the polymers could be controlled in a range of 31% to 99% by adjusting the amount of THF added. The DVP polymerization is controlled by Lu[CH(C5H4N)CH2CH2SiMe3]3 (formed via in situ mixing of Lu(CH2SiMe3)3(THF)2 and 2-vinylpyridine), with the molecular weight (Mn) matching the theoretical value of monomer conversion and a narrow dispersity. The stereoblock polymerization of DVP with 2-vinylpyridine was achieved by adding the monomer sequentially. The post-functionalization of stereoblock polymers containing vinyl groups has been achieved by the thiol–ene “click” reaction in which all the C
C bond at the 5-position selectively remained unreacted. In contrast, the polymerization of DVP by La(CH2SiMe3)3(THF)2 is not regioselective, producing a cross-linking network. The polymerization by Lu(CH2SiMe3)3(Py)2/B(C6F5)3 is most stereoselective, yielding perfect isotactic PDVP with mmmm >99%. The isoselectivity (mm) of the polymers could be controlled in a range of 31% to 99% by adjusting the amount of THF added. The DVP polymerization is controlled by Lu[CH(C5H4N)CH2CH2SiMe3]3 (formed via in situ mixing of Lu(CH2SiMe3)3(THF)2 and 2-vinylpyridine), with the molecular weight (Mn) matching the theoretical value of monomer conversion and a narrow dispersity. The stereoblock polymerization of DVP with 2-vinylpyridine was achieved by adding the monomer sequentially. The post-functionalization of stereoblock polymers containing vinyl groups has been achieved by the thiol–ene “click” reaction in which all the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C double bonds are quantitatively converted to thioether bonds.
C double bonds are quantitatively converted to thioether bonds.


 Please wait while we load your content...
Please wait while we load your content...