Thiol-reactive thiosulfonate group containing copolymers: facile entry to disulfide-mediated polymer conjugation and redox-responsive functionalizable networks†
Abstract
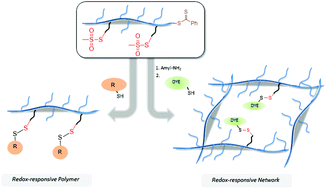
Herein, we report a synthetic approach to thiol-reactive polymers containing methanethiosulfonate groups as side chains, and demonstrate their application in post-polymerization functionalization through reversible disulfide linkages. A novel methane-thiosulfonate group containing methacrylic monomer was synthesized and copolymerized with hydrophilic poly(ethylene glycol)methyl ether methacrylate (PEGMEMA) monomer using reversible addition–fragmentation chain transfer (RAFT) polymerization. Polymers incorporating various degrees of reactive side units were obtained with narrow molecular weight distributions. The reactive thiosulfonate units on the polymer side-chains were utilized to conjugate thiol-containing molecules using the thiol-disulfide exchange chemistry. Conjugation reactions proceed in quantitative fashion at room temperature without need of any additional catalyst. Moreover, the thiosulfonate group containing polymers were utilized to fabricate thiol-reactive redox-responsive hydrogels by in situ crosslinking of copolymers in the presence of an amine-containing nucleophile. It was demonstrated that the formation of thiols via aminolysis of benzodithioate polymer end-groups resulted in the crosslinking. Facile functionalization of the resulting hydrogels containing residual methane-thiosulfonate groups was demonstrated through conjugation of a thiol-bearing fluorescent dye.


 Please wait while we load your content...
Please wait while we load your content...