Ligand- and solvent-free ATRP of MMA with FeBr3 and inorganic salts†
Abstract
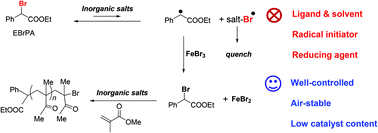
Bulk methyl methacrylate (MMA) polymerisation can be achieved with excellent control by ATRP in the presence of FeBr3/EBrPA/Mt+X−, where EBrPA = ethyl 2-bromophenylacetate and Mt+X− can be one of the several inorganic compounds (carbonate, bicarbonate, phosphate, hydroxide, chloride, bromide) of an alkali metal cation. The most effective cations are sodium and potassium. Notably, this procedure does not require the presence of any neutral ligand or coordinating solvent. The polymer chain end analysis demonstrates the initiator action of EBrPA. A mechanistic investigation shows that the ATRP activator, FeBr2, is generated in situ after EBrPA activation by the inorganic salt, deactivation of the resulting EPA radical by FeBr3, and quenching of the concurrently generated Mt+(XBr˙)− radical. This quenching occurs by the addition of this radical to MMA, but it is also possible by Fe-catalysed disproportionation when MtX = KOH. The EPA radical may also be deactivated by dimerisation and the removal of these reducing equivalents is detrimental to the FeBr2 accumulation, but the removal of the oxidizing Mt+(XBr˙)− equivalents prevails. The mechanistic investigation also confirms that the product of Br addition to MMA, methyl 1,2-dibromoisobutyrate, is not an efficient initiator for the MMA ATRP catalysed by FeBr2 under thermal conditions.


 Please wait while we load your content...
Please wait while we load your content...