Amplified photo-responses in sequentially polymerized azobenzene-containing polymer networks: the role of isomer interconnection†
Abstract
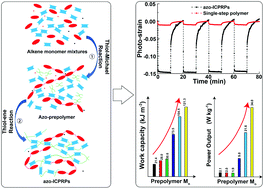
We report a one-pot synthesis method based on two-step sequential thiol–ene reactions for the facile preparation of azobenzene-interconnected photoresponsive polymers (azo-ICPRPs). In the first step, block-length-controlled azobenzene prepolymers (azo-prepolymers) are prepared in situ in a molten reaction mixture by using the nucleophile-catalyzed chemoselective thiol-Michael reaction. In the second step, the formed azo-prepolymers are further crosslinked with ultraviolet (UV)-transparent monomers by a free radical-initiated thiol–ene “click” reaction, yielding a crosslinked polymer system with rubbery storage moduli (Er) ranging from 3.6 to 6.7 MPa and glass transition temperatures (Tg) ranging from 21 to 28 °C. The interconnection of azobenzene moieties in azo-ICPRPs is found to greatly amplify the photo-actuation performance. Compared to single-step polymerized polymers with the same monomer composition, azo-ICPRPs with long-block-length showed up to 7 times higher photostrains, with 50 times faster kinetics. Consequently, the specific actuation work and power output during each stroke were also greatly amplified by the interconnection of azobenzene. The maximum measured specific stroke work and power output under 15 mW cm−2 UV activation were 121.3 kJ m−3 and 34 W kg−1, respectively, which are comparable to those for skeletal muscles.


 Please wait while we load your content...
Please wait while we load your content...