Ring opening copolymerization of ε-caprolactone and diselenic macrolide carbonate for well-defined poly(ester-co-carbonate): kinetic evaluation and ROS/GSH responsiveness†
Abstract
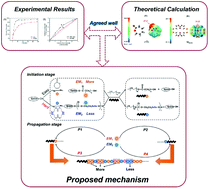
A resurgence of interest has recently propelled poly(ε-caprolactone) (PCL) back into the biomaterials arena. Herein, we reported a new poly(ester-co-carbonate) via the copolymerization of ε-CL and a diselenic macrolide carbonate monomer (MSeSe). The structure of the copolymer was confirmed by NMR, FTIR and GPC. The kinetic experimental studies showed that the monomer reactivity ratios of ε-CL (r1) and MSeSe (r2) were different and the final sequence distribution of the copolymer was random, which agreed well with the theoretical results based on density functional theory (DFT) calculations. The results of DSC and XRD consistently indicated that the copolymers were inclined to be amorphous with the increase in the MSeSe fraction. UV, DLS and TEM techniques were employed to demonstrate the variation of transmittance, size and the morphology of copolymer micelles subjected to the GSH and ROS environments, and the mechanism was clarified by FTIR and GPC, which demonstrated that the copolymers had rich environment responsiveness. Therefore, poly(ester-co-carbonate) with higher ROS and GSH sensitivity has great potential in the biomaterials field.


 Please wait while we load your content...
Please wait while we load your content...