Amphiphilic poly(esteracetal)s as dual pH- and enzyme-responsive micellar immunodrug delivery systems†
Abstract
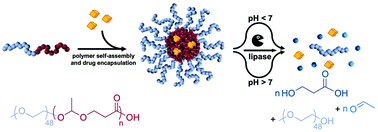
Poly(esteracetal)s are equipped with both acid-labile acetal and base-labile ester functionalities in their backbone, thus, providing dual pH-responsive degradation profiles. In this study, the cyclic esteracetal monomer 2-methyl-1,3-dioxan-4-one (MDO) was polymerised via a cationic ring-opening mechanism onto methoxy poly(ethylene glycol) (mPEG) affording amphiphilic mPEG-b-P(MDO) poly(esteracetal) block copolymers that self-assemble into well-defined monomodal micelles by direct hydration with aqueous buffers. Their pH-responsive behaviour towards rapid particle degradation was confirmed by DLS and 1H-NMR. Upon acidification or basification and lipase-mediated enzymatic hydrolysis, immediate polymer degradation and cargo release could be observed. As an example for further suitable applications, an amphiphilic immune stimulatory TLR-7 agonist (Adifectin) was co-formulated into mPEG-b-P(MDO) block copolymer micelles and its activity was investigated by TLR stimulation of RAW Blue macrophages. The degradable amphiphilic block copolymer was able to solubilize the drug more efficiently and retained its receptor activity. Consequently, mPEG-b-P(MDO) based amphiphilic poly(esteracetal)s can be considered as promising delivery systems for further immunodrugs and, thus, may contribute towards improving their translatability.
- This article is part of the themed collection: Polymer Chemistry Emerging Investigators


 Please wait while we load your content...
Please wait while we load your content...