Cleavable coumarin-based oxime esters with terminal heterocyclic moieties: photobleachable initiators for deep photocuring under visible LED light irradiation†
Abstract
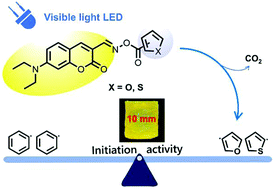
Development of efficient unimolecular visible light photoinitiators (PIs) with photobleaching capabilities, which is essential for various biomedical applications and photopolymerization of thick materials, remains an emerging challenge. Based on the photochemistry of heteroaromatic compounds, unimolecular visible light oxime ester PIs (2-O, 2-S, 3-O, and 3-S), which contain coumarin derivatives as visible light chromophores, were designed and synthesized. Photophysical and photochemical properties of these PIs have been investigated using UV-Vis spectroscopy, fluorescence spectroscopy and electron spin resonance (ESR). The oxime-esters photolyse rapidly under 450 nm LED light irradiation, especially 2-S, and have good photobleaching capabilities. Also, we have, for the first time, shown that heterocyclic radicals efficiently initiate free radical polymerization of acrylate monomers. Notably, these photobleaching initiators can be used to induce thiol-based click photopolymerization to prepare materials with a thickness of up to 10 mm.


 Please wait while we load your content...
Please wait while we load your content...