Preparation of hyperbranched polymers by oxa-Michael addition polymerization†
Abstract
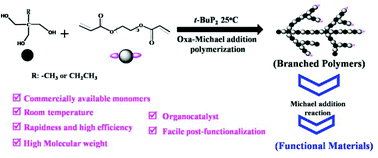
In this research, we developed an efficient approach to prepare hyperbranched polymers at room temperature via phosphazene-base t-BuP2 catalyzed oxa-Michael addition polymerization from commercially available trifunctional hydroxyl and diacrylate monomers. The branching structure of the obtained polymers and the polymerization process were investigated by nuclear magnetic resonance (NMR) spectroscopy and triple-detection size-exclusion chromatography (TD-SEC) analysis. It was revealed that acrylic double bond terminated branched polymers with high molecular weights and high degrees of branching (Mw.MALLS > 2.8 × 105 g mol−1, DB ≥ 0.8) were produced by t-BuP2 catalyzed oxa-Michael addition polymerization of a trimethylolpropane (TMP) with a double molar 1,6-hexanediol diacrylate (HDDA) in DMF at room temperature, even at 0 °C. The study of the branching process showed that t-BuP2 catalyzed oxa-Michael addition branching polymerization is rapid, and that significant branched structures formed when the polymerization was performed at 3 min. Most importantly, the prepared branched polymers can be further post-functionalized via aza- or thio-Michael addition reactions, due to the polymers retaining the acrylic double bond functionality. This research provides a versatile and efficient method for the preparation of hyperbranched polymers from commercially available monomers, and it is feasible to prepare functional branched polymers for application in various fields.


 Please wait while we load your content...
Please wait while we load your content...