1 : 1 alternating and 1 : 2 sequence-controlled radical copolymerization of 1,3-pentadiene isomers with maleic anhydride/N-phenylalkyl maleimide†
Abstract
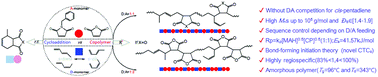
The competition between the Diels–Alder cycloaddition and the sequence-controlled radical copolymerization of maleic anhydride/N-phenylalkyl maleimide (MAH/NPMI) with 1,3-pentadiene (PD) isomers was investigated. The copolymerization behavior significantly depended on the isomer conformation as well as the feeding ratio, leading to the formation of AB-alternating and AAB-periodic (2 : 1 sequence-controlled) copolymers with Mn values up to 105 g mol−1 under mild conditions. The sequence-controlled radical copolymerization mechanism was discussed based on the “bond-forming initiation theory” via the key tetramethylene-intermediate, which is in agreement with the results of the kinetics analysis and the microstructure analysis determined by NMR. The suggested mechanism involved the formation of a π-allyl-2-hexene 1,6-diradical formed by the reaction of s-trans PD with MAH, which initiates the MAH-CP alternating copolymerization.


 Please wait while we load your content...
Please wait while we load your content...