Lewis pair catalyzed highly selective polymerization for the one-step synthesis of AzCy(AB)xCyAz pentablock terpolymers†
Abstract
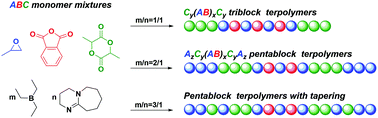
A key challenge in polymer chemistry is to regulate the polymer sequence and architecture from versatile monomer mixtures. Here we describe the triethylborane/1,8-diazabicyclo[5.4.0]undec-7-ene (Et3B/DBU) pair catalysed highly selective terpolymerization of mixtures of propylene oxide (PO), phthalic anhydride (PA) and racemic lactide (rac-LA), wherein the catalytic efficiency and the polymerization selectivity are significantly correlated to the Et3B/DBU feed ratio. With H2O as a cheap and efficient initiator, the Et3B/DBU pair (1/1) demonstrates chemoselective control over the ring-opening copolymerization (ROCOP) of PO/PA and the ring-opening polymerization (ROP) of rac-LA, while the Et3B/DBU pair (2/1) additionally allows for the discrimination of rac-LA and PO in ROP via kinetic control, affording one-step access to unique AzCy(AB)xCyAz pentablock terpolymers (Đ < 1.15) without transesterification scrambling.


 Please wait while we load your content...
Please wait while we load your content...