Synthesis of biodegradable protein–poly(ε-caprolactone) conjugates via enzymatic ring opening polymerization†
Abstract
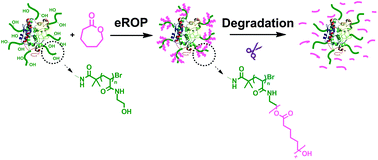
Nondegradable PEGylated protein drugs are known to cause accumulation in the tissue and accelerated blood clearance effect, which inspire people to develop alternative polymers such as polyesters for bioconjugation. However, the hydrophobicity and slow degradation rate of polyesters remain as challenges for typical therapeutic applications. Here, we report the facile synthesis of biodegradable protein–poly(ε-caprolactone) conjugates via enzymatic ring opening polymerization (eROP). Candida antarctica lipase B (CALB)–poly(N-hydroxyethyl acrylamide) conjugates with pendent hydroxyl groups were utilized as initiators and catalysts simultaneously for the eROP of ε-caprolactone to form amphiphilic graft copolymers. The enzymatic degradation of ester-containing polymers in the conjugates could be significantly accelerated by lipase, leading to total degradation in several days.


 Please wait while we load your content...
Please wait while we load your content...