Copolymers of ε-caprolactone and ε-caprolactam via polyesterification: towards sequence-controlled poly(ester amide)s†
Abstract
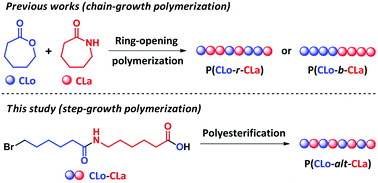
Poly(ester amide)s (PEAs) attract considerable attention owing to their intriguing properties. However, the establishment of precise structure–property relationship for PEAs is hindered by the ambiguous primary structures of these biologically important polymers. Herein, we propose polyesterification as an efficient strategy for the synthesis of copolymers of ε-caprolactone (CLo) and ε-caprolactam (CLa). Two sorts of AB-type monomers bearing halogen and carboxylic acid terminal groups, namely 6-bromohexanoic acid (1) and 6-(6-bromohexanamido)hexanoic acid (2), underwent nucleophilic substitution in a step-growth manner to furnish P(CLo) (P0) and P(CLo-alt-CLa) (P5), respectively. Moreover, random copolymers P1–P4 were also synthesized by copolymerization of 1 and 2 in predetermined feed ratios. Thermal properties, crystal structure, surface wettability, thermal responsiveness and degradation profiles of P0–P5 along with P(CLa) (P6) were investigated. The melting ranges of polymers P0–P6 become narrowed if the structural regularity is pronounced, and the melting temperatures (Tms) are fundamentally determined by chemical composition and sequence distribution. The crystal structures and morphologies of P0–P6 are influenced by their chemical compositions, and the unit cell changes from orthorhombic to α-monoclinic while increasing the molar fraction of the CLa unit. Moreover, alternating copolymer P5 shows upper critical solution temperature (UCST) behavior in dimethyl sulfoxide (DMSO).


 Please wait while we load your content...
Please wait while we load your content...