Ethylene free radical polymerization in supercritical ethylene/CO2 mixture†
Abstract
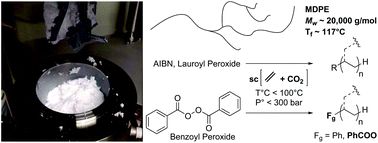
Polymerization of ethylene via radical pathways involving high temperatures and pressures (>200 °C, >2000 bar) is the current process of choice to produce low-density polyethylene (LDPE). Here we report ethylene free radical polymerization in supercritical CO2/ethylene mixture as a reaction medium without any addition of organic solvent and under milder conditions (<100 °C, <300 bar). The effect of CO2 pressure on the yield and the molar masses of the polyethylenes (PE) produced is substantial and depends on the chosen initiator. The three selected initiators (AzobisIsoButyroNitrile, lauroyl peroxide, and benzoyl peroxide) all yield PE, with Mw up to ∼20 000 g mol−1, confirming the non-transferring nature of CO2. We also investigated the divergences in initiation/transfer/termination for these 3 compounds embedded within the functionalities borne by the polyethylene chains, and in particular the influence of CO2 over the polymerization. No CO2-derived moieties could be evidenced within the PE chains, neither for AIBN nor lauroyl peroxide. On the contrary, benzoic ester moieties could be identified within the produced PE when benzoyl peroxide was employed. This study paves the road towards functionalization of PE via radical processes.


 Please wait while we load your content...
Please wait while we load your content...