Self-crosslinking assemblies with tunable nanostructures from photoresponsive polypeptoid-based block copolymers†
Abstract
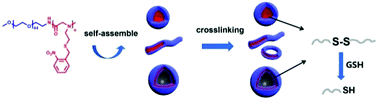
Photoresponsive polymers have been receiving tremendous attention for many applications. Here, we report a new family of photoresponsive polypeptoid-based diblock copolymers PEG-b-poly(N-(S-(o-nitrobenzyl)-thioethyl) glycine) (PEG-b-PNSN) by ring-opening polymerization (ROP). The polymerization is well-controlled and a series of copolymers have been obtained with narrow polydispersity. We demonstrate that the cleavage degree of the o-nitrobenzyl (NB) group can reach 73% with the irradiation time increasing up to 6 hours. To the best of our knowledge, this is the first example of photoresponsive polypeptoids prepared by ROP. Depending on the chain length of PNSN, the PEG-b-PNSN diblock copolymers can self-assemble into various morphologies, including spheres, short cylinders and vesicles. More importantly, the thiol groups generated by UV-irradiation can be spontaneously oxidized into disulfide bonds, which behave as cross-linkers to stabilize the nanostructures with constant morphologies. Furthermore, this oxidation process is reversible in the presence of the reductive reagent glutathione (GSH), resulting in reversible self-crosslinking assemblies. The obtained photoresponsive polypeptoid copolymers are ideal candidates for smart polymeric materials in applications of nanomedicine and nanotechnology.
- This article is part of the themed collection: Polymer Chemistry Emerging Investigators


 Please wait while we load your content...
Please wait while we load your content...