Radical anion formation exhibiting “turn-on” fluorescence sensing of hydrazine using a naphthalene diimide (NDI) derivative with a donor–acceptor–donor (D–A–D) molecular structure†
Abstract
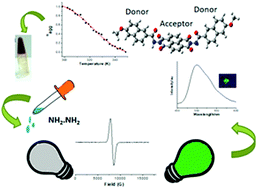
In this paper, the synthesis of a naphthalene diimide (NDI) derivative with a donor–acceptor–donor (D–A–D) molecular structure substituted with a long alkyl chain (12 carbons) containing naphthalene hydrazide at the imide position is reported. The reduced emission quantum yield (ϕf = 0.01–0.03) of the NDI derivative in various solvents indicates the perturbation of the electronic state of π-electron deficient NDI (A) by the peripheral naphthalene (D) units. The investigation of the influence of the alkyl chain and naphthalene substituent on the self-assembling properties of the NDI derivative reveals an isodesmic mode of self-assembly in a chloroform/methylcyclohexane (CHCl3/MCH, 1 : 9, v/v) mixture. The self-assembling nature of the NDI derivative also results in the formation of an organogel in the CHCl3/MCH (1 : 9, v/v) mixture, and gel formation is well-comprehended by techniques such as P-XRD, rheological studies, and FT-IR measurements. Furthermore, radical anion (NDI˙−) formation of π-acidic NDI was used as a sensing tool for hydrazine by a fluorescence “turn-on” (ϕf = 0.12) method in the solution (DMSO), film, and gel state with a detection limit of 284.1 ppb in DMSO and 32 ppb in the gel state.


 Please wait while we load your content...
Please wait while we load your content...