Heck reaction synthesis of anthracene and naphthalene derivatives as traps and clean chemical sources of singlet molecular oxygen in biological systems
Abstract
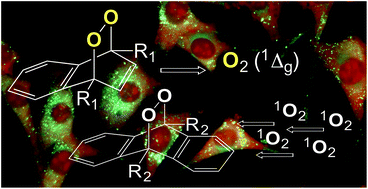
Studies have previously shown that anthracene and naphthalene derivatives serve as compounds for trapping and chemically generating singlet molecular oxygen [O2(1Δg)], respectively. Simple and efficient synthetic routes to anthracene and naphthalene derivatives are needed, for improved capture and release of O2(1Δg) in cellular environments. Because of this need, we have synthesized a dihydroxypropyl amide naphthlene endoperoxide as a O2(1Δg) donor, as well as five anthracene derivatives as O2(1Δg) acceptor. The anthracene derivatives bear dihydroxypropyl amide, ester, and sulfonate ion end groups connected to 9,10-positions by way of unsaturated (vinyl) and saturated (ethyl) bridging groups. Heck reactions were found to yield these six compounds in easy-to-carry out 3-step reactions in yields of 50–76%. Preliminary results point to the potential of the anthracene compounds to serve as O2(1Δg) acceptors and would be amenable for future use in biological systems to expand the understanding of O2(1Δg) in biochemistry.


 Please wait while we load your content...
Please wait while we load your content...