Aryl dechlorination and defluorination with an organic super-photoreductant†
Abstract
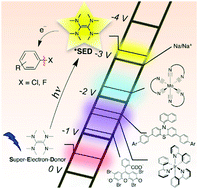
Direct excitation of the commercially available super-electron donor tetrakis(dimethylamino)ethylene (TDAE) with light-emitting diodes at 440 or 390 nm provides a stoichiometric reductant that is able to reduce aryl chlorides and fluorides. The method is very simple and requires only TDAE, substrate, and solvent at room temperature. The photoactive excited state of TDAE has a lifetime of 17.3 ns in cyclohexane at room temperature and an oxidation potential of ca. −3.4 V vs. SCE. This makes TDAE one of the strongest photoreductants able to operate on the basis of single excitation with visible photons. Direct substrate activation occurs in benzene, but acetone is reduced by photoexcited TDAE and substrate reduction takes place by a previously unexplored solvent radical anion mechanism. Our work shows that solvent can have a leveling effect on the photochemically available redox power, reminiscent of the pH-leveling effect that solvent has in acid–base chemistry.


 Please wait while we load your content...
Please wait while we load your content...