Does the degree of substitution on the cyclodextrin hosts impact their affinity towards guest binding?†
Abstract
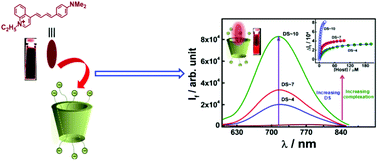
Although cyclodextrins have been extensively utilized in various branches of supramolecular chemistry due to their numerous attractive attributes, however, to achieve even advanced applications, they often need structural modification through substitutions of suitable functional groups at their rims. A systematic investigation on how the degree of substitution on the cyclodextrin rims affects the binding affinity for a given guest molecule has however rarely been reported, especially from the perspective of photophysical studies. Herein, we report the non-covalent interaction of a styryl based dye, LDS-798, with three different sulfobutylether beta cyclodextrin (SBEnβCD) derivatives bearing varying degrees of substitution (n), using ground state absorption, steady-state emission, excited-state lifetime and time-resolved fluorescence anisotropy measurements. The dye–host binding constant values indicate that the strength of the interaction between LDS-798 and SBEnβCD derivatives follows an increasing trend with an increasing number of tethered sulfobutylether substituents on the cyclodextrin rims, which is attributed to the gradual increase of the electrostatic interaction between the negatively charged sulfobutylether groups and the positively charged LDS-798. Excited state lifetime measurements and ionic strength dependent studies on the dye–SBEnβCD complexes further support the increased affinity between the dye and the host in the supramolecular complexes, with an increasing number of sulfobutylether substituents on the βCD rims. The obtained results suggest that the molecular recognition of LDS-798 with SBEnβCD derivatives can be tuned very effectively by varying the number of sulfobutylether substituents on the cyclodextrin rims. Considering that SBE7βCD is one of the FDA approved agents for drug formulations, the obtained results with other SBEnβCD hosts may be useful in designing selective drug delivery applications, drug formulations, and effective fluorescence on–off switches.


 Please wait while we load your content...
Please wait while we load your content...