Understanding the role played by protic ionic liquids (PILs) and the substituent effect for enhancing the generation of Z-cinnamic acid derivatives†
Abstract
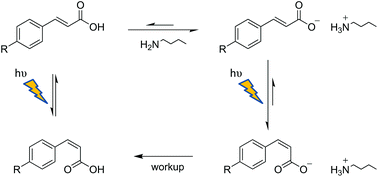
Photoisomerization of a series of substituted E-cinnamic acids in MeCN in their acid forms and as their corresponding protic ionic liquids (PILs) with light of 300 nm is studied. The nature, strength, number, and position effects of substituents on the photochemical behavior of E-cinnamic derivatives are investigated. The photosensitization of the reaction in the presence of Michler's ketone is also studied at 366 nm and it demonstrates that the triplet-excited state is involved in the reaction. As the presence of n-butylamine needed to form the PILs significantly increases the photoproduct yields in all cases, the role of the PILs is also discussed. Thus, understanding of these fundamental aspects has allowed us to establish an excellent and practical synthetic protocol for successfully synthesizing Z-cinnamic acids.


 Please wait while we load your content...
Please wait while we load your content...