Turn-on mode fluorescent diarylethenes: effect of electron-donating and electron-withdrawing substituents on photoswitching performance†
Abstract
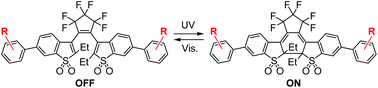
Diarylethene derivatives having benzothiophene S,S-dioxide groups undergo turn-on mode fluorescence photoswitching. For the practical application to super-resolution fluorescence microscopy, photoswitchable fluorescent molecules are desired to be resistant against photodegradation. Here we synthesized turn-on mode fluorescent diarylethenes having electron-withdrawing (trifluoromethyl or nitro) or electron-donating (methyl, methoxy, or dimethylamino) substituents on phenyl rings at 6- and 6′-positions of the benzothiophene S,S-dioxide groups and examined the effect of the substituents on the photoswitching performance. The derivatives having electron-donating substituents showed significant bathochromic shifts of the absorption and fluorescence spectra. The cycloreversion quantum yield was increased by introducing electron-withdrawing substituents, while it was decreased by the electron-donating ones. Introduction of electron-donating substituents was found to remarkably improve the fatigue resistance of the fluorescent diarylethene under continuous ultraviolet (UV) irradiation. Such highly fatigue-resistant fluorescent diarylethenes are useful for super-resolution fluorescence imaging or single-molecule fluorescence tracking.


 Please wait while we load your content...
Please wait while we load your content...