The origin of the longer wavelength emission in 2-(4-fluorophenylamino)-5-(2,4-dihydroxybenzeno)-1,3,4-thiadiazole and its analogue 2-phenylamino-5-(2-hydroxybenzono)-1,3,4-thiadiazole†‡
Abstract
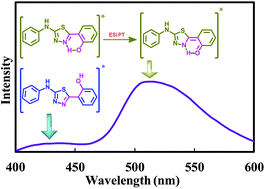
In aqueous solution, 2-(4-fluorophenylamino)-5-(2,4-dihydroxybenzeno)-1,3,4-thiadiazole (FABT) was found to emit dual emission and the longer wavelength emission was assigned to the combination of aggregation and conformational change. In a number of molecules that possess an intramolecular hydrogen bond between the proton donor and the acceptor, the longer wavelength emission is often observed due to the emission from the tautomer formed by excited state intramolecular proton transfer (ESIPT). Therefore, an analogue of FABT, 2-phenylamino-5-(2-hydroxybenzono)-1,3,4-thiadiazole (PHBT), was synthesized to determine the origin of the longer wavelength emission. The luminescence of PHBT and its methoxy derivatives was studied and compared with that of FABT. Theoretical calculations were also performed on both FABT and PHBT. Based on the experimental and theoretical investigations, the nonexistence of the keto tautomer in the ground state and the origin of the longer wavelength emission are divulged.


 Please wait while we load your content...
Please wait while we load your content...