Enantiospecific deoxyfluorination of cyclic α-OH-β-ketoesters†
Abstract
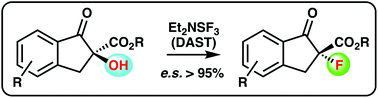
We herein report the deoxyfluorination of cyclic α-hydroxy-β-ketoesters using diethylaminosulfur trifluoride (DAST). The reaction proceeds with excellent levels of stereospecificity, giving the configurationally inverted α-fluoro-β-ketoesters in high yields under operationally simple conditions.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...