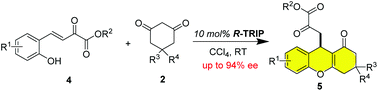
Yu-Qi Gao, Yi Hou, Junhan Chen, Yanxia Zhen, Dongyang Xu, Hongli Zhang, Hongbo Wei and Weiqing Xie
Org. Biomol. Chem., 2021,19, 348-354
DOI:
10.1039/D0OB02140G,
Communication