Conversions of aryl carboxylic acids into aryl nitriles using multiple types of Cu-mediated decarboxylative cyanation under aerobic conditions†
Abstract
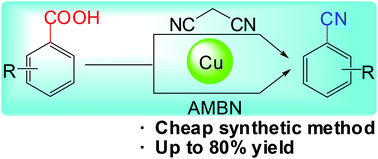
Here, we used malononitrile or AMBN as a cyanating agent to develop efficient and practical protocols for Cu-mediated decarboxylative cyanations, under aerobic conditions, of aryl carboxylic acids bearing nitro and methoxyl substituents at the ortho position as well as of heteroaromatic carboxylic acids. These protocols involved economical methods to synthesize value-added aryl nitriles from simple and inexpensive raw materials. Further diversification of the 2-nitrobenzonitrile product was performed to highlight the practicality of the protocols.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...