Kinase and GPCR polypharmacological approach for the identification of efficient anticancer medicines†
Abstract
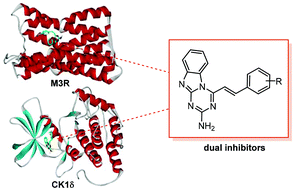
Discovery of an anticancer medicine using a single target protein has often been unsuccessful due to the complexity of pathogenic mechanisms as well as the presence of redundant signaling pathways. In this work, we attempted to find promising anticancer drug candidates by simultaneously targeting casein kinase 1 delta (CK1δ) and muscarinic acetylcholine receptor M3 (M3R). Through the structure-based virtual screening and de novo design with the modified potential function for protein–ligand binding, a series of benzo[4,5]imidazo[1,2-a][1,3,5]triazine-2-amine (BITA) derivatives were identified as CK1δ inhibitors and also as M3R antagonists. The biochemical potencies of these bifunctional molecules reached the nanomolar and low-micromolar levels with respect to CK1δ and M3R, respectively. A common interaction feature in the calculated CK1δ-inhibitor and M3R-antagonist complexes is that the BITA moiety is well-stabilized in the orthosteric site of M3R and the hinge region of CK1δ through the establishment of the three hydrogen bonds and the hydrophobic contacts in the vicinity. The computational and experimental results found in this work exemplify the efficiency of kinase and GPCR polypharmacology in developing anticancer medicines.


 Please wait while we load your content...
Please wait while we load your content...