Ruthenium-catalyzed α-carbonyl sulfoxonium ylide annulations with aryl substituted pyrazoles via C–H/N–H bond functionalizations†
Abstract
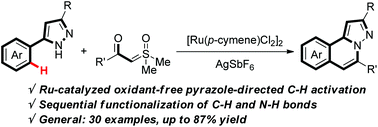
An efficient method for the construction of various pyrazolo[5,1-a]isoquinolines has been achieved via Ru(II)-catalyzed α-carbonyl sulfoxonium ylide annulations with aryl substituted pyrazoles. This oxidant-free transformation occurred through pyrazole-directed C–H activation, Ru-carbene insertion, and acid-promoted carbonyl condensation processes, enabling dual C–C and C–N bond formation. A broad substrate scope with respect to both coupling components worked efficiently with high yields.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...