NaHSO4/SiO2 catalyzed generation of o-quinone/ o-thioquinone methides: synthesis of arylxanthenes/ arylthioxanthenes via oxa-6π-electrocyclization†
Abstract
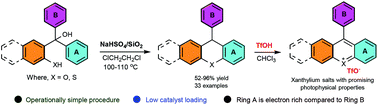
ortho-Quinone methides, very reactive transient intermediates, are utilized successfully in synthesizing complex organic molecules of natural and biological significance. Among several synthetic protocols, the acid catalyzed generation of ortho-quinone methides from suitably substituted phenols is a promising method for further exploitation in organic synthesis. Such an interesting reactive species is conveniently employed in the synthesis of conformationally restricted triarylmethane derivatives such as 12/9-arylxanthenes/arylthioxanthenes starting from symmetrical/unsymmetrical 2-(hydroxydiarylmethyl)phenol/thiophenol, respectively, using SiO2–NaHSO4. Conformationally restricted 12/9-arylxanthenes/arylthioxanthenes were obtained in 52 to 96% yields using this protocol, which is believed to involve the formation of o-quinone methides followed by electrocyclic ring closure and isomerization at elevated temperature. Photophysical studies of selected examples in acidic media showed turn-on fluorescence by hydride ion transfer mediated π-conjugated xanthylium salt formation and suggested the application potential in bio-imaging and fluorescent sensors.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...